www.moonlandings.co.uk
The Moon | Radiation & Van Allen Belts | A Void of Space | Space Cameras | Temperature | Photograph Anomolies
Radiation & Van Allen Belts

Introduction to Radiation
Heat and light are types of radiation that people can feel or see, but there are other kinds of radiation that human senses cannot detect. Indeed, we constantly receive such invisible radiation from the sky, the ground, the air, and even our food and drink. Such 'ionizing' radiation has been put to many uses: doctors use X rays to diagnose disease or injury; factories use radiation to check welds in machine components; gamma rays are used to sterilise medical equipment for safe use; and many new varieties of crops have been produced through radiation-induced mutations. Today moreover, about 17 percent of the world's electricity is supplied by nuclear power plants. The usefulness of radiation means that many people receive small doses of radiation from artificial sources as well as doses from nature.
Ionizing radiation from radioactive materials diminishes over time at various rates as the atoms change into other atoms. Often, there is not just the disappearance of one kind of radiation, but the production of different radiations if the new atoms are radioactive. The time for half the radioactivity to dissipate is called the 'half-life'. Half-lives vary from a small fraction of a second to many millions of years.
Measuring Radiation
The amount of radiation the 'dose' received by people is measured in millisieverts (mSv). The unit is the sievert, but millisieverts (mSv) are commonly used. This unit belongs to the same family as the litre and kilogram, the most commonly accepted, international system of units. For radiation protection purposes, exposure to ionizing radiation is most often measured in terms of 'effective dose'. This is based on the energy deposited in tissue by radiation, taking into account the type of radiation and the sensitivity of the tissues irradiated. It is thus a measure of the overall risk arising from the exposure.
Sources of Natural Radiation
Everyone is exposed to radiation, and for most people nature is the largest source of exposure. For purposes of this Web site, only those that affect astronauts and equipment outside the cofines of the Eart's atmosphere are looked at, although other sourses are listed here for the casual reader to inform him/herself.
Cosmic radiation comes through the earth's atmosphere, some from the sun and energy sources in our galaxy or outside it. Those from the sun are more intense during solar flares but the others are fairly constant in number. However, the density is affected by the earth's magnetic field, which makes it greater nearer the poles than the equator. The radiation dose people receive increases therefore with latitude. In addition, the earth's atmosphere is a partial shield to the radiation. As one goes higher there is a lower shielding effect and the dose increases as the altitude increases. Buildings and the fuselages of aircraft provide little protection. (Source) The global yearly average dose is 0.39 millisieverts.
The Van Allen Belts
For astronauts, the biggest threat to health and efficiency of equipment are the Van Allen Belts. A visual 'demonstation' of radiation surrounding the Earth are the aurora borealis and aurora australis; thought to be caused by high-speed electrons and protons from the sun, trapped in the Van Allen radiation belt high above the Earth and then channeled toward the polar regions by the Earth's magnetic field.
Physically, the Van Allen radiation belts are two 'belts' of high-energy charged particles of radiation (sometimes considered as a single belt of varying intensity) outside the Earth's atmosphere, extending from around 650 to 65 000 km above the Earth. Their existence was confirmed from information secured by launching the first US Earth satellite, Explorer I, sent up during the International Geophysical Year of 1957 - 58. The belts were named after James A Van Allen, the American astrophysicist who first predicted the belts and then was first to interpret the findings of the Explorer satellite. The region of external belts has been named the magnetosphere to distinguish it from the atmosphere. The charged particles of which the belts are composed circulate along the Earth's magnetic lines of force extending from the area above the equator to the North Pole, to the South Pole, and circles back to the equator. These particles are believed to originate in periodic solar flares. Carried by the solar wind, they become trapped by the Earth's magnetic field and are responsible for the aurora borealis (as below) seen at polar regions. A part of a belt dips into the upper region of the atmosphere over the southern Atlantic Ocean to form the  Southern Atlantic Anomaly (a region through which low-orbiting satellites frequently pass). This is a region of very high particle flux about 250 km above the Atlantic Ocean off the coast of Brazil and is a result of the fact that the Earth's rotational and magnetic axes are not aligned (further ref). The particle flux is so high in this region that often the detectors on our satellites must be shut off (or at least placed in a 'safe' mode) to protect them from the radiation.
Southern Atlantic Anomaly (a region through which low-orbiting satellites frequently pass). This is a region of very high particle flux about 250 km above the Atlantic Ocean off the coast of Brazil and is a result of the fact that the Earth's rotational and magnetic axes are not aligned (further ref). The particle flux is so high in this region that often the detectors on our satellites must be shut off (or at least placed in a 'safe' mode) to protect them from the radiation.
The inner belt, located between about X = 1.1 - 3.3 Re (Earth radii, geocentric) in the equatorial plane, contains primarily protons with energies exceeding 10 MeV. Flux maximum is at about X = 2 Re. (Distances given here are approximate, since the location of particles is energy dependent). This is a fairly stable population but it is subject to occasional perturbations due to geomagnetic and varies with 11-year solar cycle. The source of protons in this region is the decay of cosmic ray induced albedo from the atmosphere. Energetic particles in this region can be a source of problems for the satellites and astronauts. It consists mainly a high-energy protons (10 - 50 MeV) and is a by-product of the cosmic radiation, a thin drizzle of very fast protons and nuclei which apparently fill all our galaxy. In addition there exist electrons and protons (and also oxygen particles from the upper atmosphere) given moderate energies (say 1-100 keV; 1 MeV = 1000 keV) by processes inside the domain of the Earth's magnetic field. Some of these electrons produce the polar aurora when they hit the upper atmosphere, but many get trapped, and among those, protons and positive particles have most of the energy.
A typical satellite passing the radiation belts (elliptic orbit 320 km to 32000 km) would have an annual radiation dosage of about 2500 rem, assuming one is shielded by 1 gr/cm-square of aluminum (around 3mm thick plate) - almost all of it while passing the inner belt. But there is no danger to humans on Earth. The way the particles move in the magnetic field prevents them from hitting the atmosphere, and even if they are scattered so their orbit does intersect the ground, the atmosphere absorbs them long before they get very far. Even the space station would be safe, because the orbits usually stop below it - any particles dipping deeper down are lost much faster than they can be replenished (further info.)
The outer belt contains mainly electrons with energies up to 10 MeV. It is produced by injection and energization events following geomagnetic storms, which makes it much more dynamic than the inner belt (it is also subject to day-night variations). It has an equatorial distance of about 3 - 9 Re, with maximum for electrons above 1 MeV occurring at about X = 4 Re. 'Horns' of the outer belt dip sharply in towards the polar caps.
In 1998 a new belt was found within the inner belt. It contains heavy nuclei (mainly oxygen, but also nitrogen and helium, and very little carbon) with energies below 50 MeV/nuc. The source of these particles are the so called 'anomalous cosmic rays' of interstellar origin.
The radiation belts are of importance primarily because of the harmful effects of high energy particle radition for man and electronics:
The investigation of the Earth's radiation environment was one of the main tasks of the CRRES satellite. It has observed, for example, a rapid (1 min) formation of a new radiation belt due to an SSC on 24 March 1991.

Time magazine of 4 May 1959 credited James Van Allen as the man most responsible for giving the US “a big lead in scientific achievement.” They called Van Allen “a key figure in the cold war’s competition for prestige." Today he can tip back his head and look at the sky. Beyond its outermost blue are the world-encompassing belts of fierce radiation that bear his name. No human name has ever been given to a more majestic feature of the planet Earth
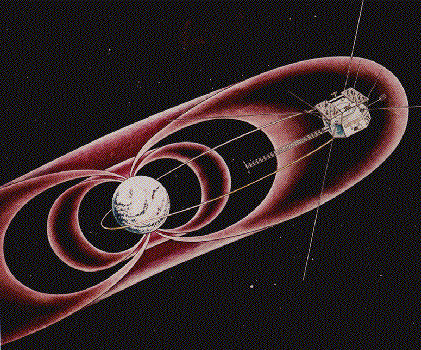
A depiction of CRRES GTO orbit through toroidal Van Allen Radiation Belt, showing Geosynchronous Transfer Orbit (GTO)
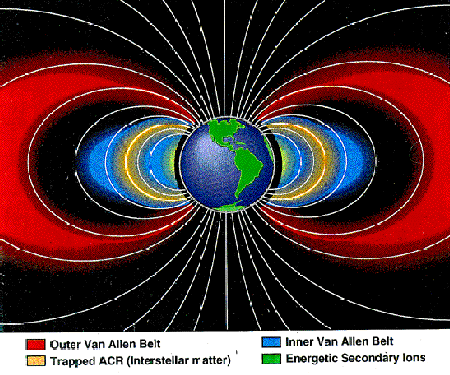
The electrons and ions in the Van Allen belts are effectively trapped in Earth's magnetic dipole field. Bouncing between hemispheres, they reverse their motion at their closest approach to Earth, while at the same time drifting around it. If their trajectories take them too deeply into the atmosphere where they collide with the ambient particles and lose their energy, they are lost from the radiation belts. In this figure, 'trapped ACR' refers to ions that originate in the interstellar gas, while energetic secondary ions originate from collisions of cosmic rays with atmospheric gases
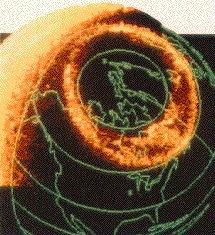 This image of the auroral oval shows the bright emissions that occur in the atmosphere at altitudes between 80 and 300 kilometres when atoms are excited by energetic electrons travelling along magnetic field lines. This image was obtained from the Dynamics Explorer spacecraft over the north polar region during winter, when the entire aurora borealis was over the night hemisphere of the Earth (from: Dynamics Explorer University of Iowa Imaging Experiment)
This image of the auroral oval shows the bright emissions that occur in the atmosphere at altitudes between 80 and 300 kilometres when atoms are excited by energetic electrons travelling along magnetic field lines. This image was obtained from the Dynamics Explorer spacecraft over the north polar region during winter, when the entire aurora borealis was over the night hemisphere of the Earth (from: Dynamics Explorer University of Iowa Imaging Experiment)The aurora borealis is said to occur with greatest frequency along a line extending through North Norway, across central Hudson Bay, through Point Barrow, Alaska, and through northern Siberia. It is often visible in Canada and northern United States and is seen most frequently at the time of the equinoxes. Among the most magnificent of natural phenomena, auroral displays appear in shades of red, yellow, green, blue and violet and are usually brightest in their most northern latitudes. The aurora is seen in a variety of forms, eg as patches of light, in the form of streamers, arcs, banks, rays or resembling hanging draperies. The aurora occurs between 56 km and 970 km above the Earth. It is thought to be caused by high-speed electrons and protons from the sun, which are trapped in the Van Allen radiation belt high above the Earth and then channeled toward the polar regions by the Earth's magnetic field. These electrically charged particles enter the atmosphere and collide with air molecules (chiefly oxygen and nitrogen), thus exciting them to luminosity; near the 900 km level the light may be given off by electrons and protons combining to form hydrogen atoms. The auroras coincide with periods of greatest sunspot activity and with magnetic storms (disturbances of the ionosphere which interfere with long-distance radio communication). Much was learned about the aurora during the 1957–58 International Geophysical Year, when it was studied intensively by means of balloons, radar, rockets and satellites.


Above: The aurora as seen from Earth Right top:NASA photo showing aurora from space
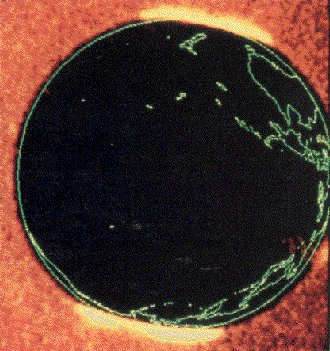

Above: The aurora borealis ('northern lights') and the aurora australis ('southern lights') as glowing regions at each pole as seen by NASA's Dynamics Explorer satellite
Right: The aurora borealis as seen from the ground. Different colours arise because different atmospheric gases are excited, and the excitation occurs at different altitudes as a result of the wide energy spread of the exciting electrons
Other Sources of Natural Radiation
The Earth's Crust is made up of materials that are naturally radioactive. Uranium, for instance, is dispersed throughout rocks and soil, mostly at very low concentrations. So are thorium and potassium-40. They nearly all emit gamma rays which irradiate the whole body more or less uniformly. Since building materials are extracted from the earth, they can be slightly radioactive, and people are irradiated indoors as well as out of doors. The radiation doses vary according to the rocks and soils of the area and the building materials in use but the global yearly average is 0.46 millisieverts.
Radon is a naturally radioactive gas that comes from the uranium that is widespread in the earth's crust. It is emitted from rocks or soil at the earth's surface and disperses in the atmosphere unless it enters a building, in which the concentration can build up. Radon decays to form other radioactive atoms which, when inhaled, can lodge in the lung and irradiate tissue. The global yearly average dose is 1.3 millisieverts but in high radon areas the doses can be many times higher. The radiation dose can most easily be reduced by preventing the radon gas from entering it in the first place.(see Fig.3)
Food and Drink. Since radioactive materials occur everywhere in nature it is inevitable that they get into drinking water and food, giving a global yearly average dose of 0.23 millisieverts. Potassium-40 in particular is a major source of internal irradiation, but there are others. Potassium-40 in the body varies with the amount of muscle, for instance, being twice as high in younger men than in older women. Some foods, for example shellfish and Brazil nuts, concentrate radioactive materials so that, even when there is no artificial radioactivity, people who consume large quantities can receive a radiation dose significantly above average.
Sources of Artificial Radiation
Doses from artificial radiation are, for most of the population, much smaller than those from natural radiation but they still vary considerably. They are in principle fully controllable, unlike natural sources.
Medical. Radiation is used in medicine in two distinct ways: to diagnose disease or injury; and to kill cancerous cells. In the oldest and most common diagnostic use, X rays are passed through the patient to produce an image. The technique is so valuable that millions of X ray examinations are conducted every year. One chest X ray will give 0.1 mSv of radiation dose. For some diseases, diagnostic information can be obtained using gamma rays emitted by radioactive materials introduced into the patient by injection, or by swallowing or by inhalation. This technique is called nuclear medicine. The radioactive material is part of a pharmaceutical chosen so that it preferentially locates in the organ or part of the body being studied. To follow the distribution or flow of the radioactive material a gamma camera is used. It detects the gamma radiation and produces an image, and this indicates whether the tissue is healthy or provides information on the nature and extent of the disease.
Cancerous conditions may be treated through radiotherapy, in which beams of high energy X rays or gamma rays from cobalt-60 or similar sources are used. They are carefully aimed to kill the diseased tissue, often from several different directions to reduce the dose to surrounding healthy tissue. Radioactive substances, either as small amounts of solid material temporarily inserted into tissues or as radioactive solutions, can also be used in treating diseases, delivering high but localised radiation doses.
Medical uses of radiation are by far the largest source of man-made exposure of the public; the global yearly average dose is 0.3 millisieverts.
Environmental Radiation. Radioactive materials are also present in the atmosphere as a result of atomic bomb testing and other activities. They may lead to human exposure by several pathways external irradiation from radioactive materials deposited on the ground; inhalation of airborne radioactivity, and ingestion of radioactive materials in food and water.
Radioactive fall-out from nuclear weapons tests carried out in the atmosphere is the most widespread environmental contaminant but doses to the public have declined from the relatively high values of the early 1960s to very low levels now. The global yearly average dose is 0.006 millisieverts. However, where tests were carried out at ground level or even underground, localised contamination often remains near weapons sites.
Nuclear and other industries, and to a small degree hospitals and universities, discharge radioactive materials to the environment. Nearly all countries regulate industrial discharges and require the more significant to be authorized and monitored. Monitoring of such effluent may be carried out by the government department that authorizes the discharges as well as by the operator.
The nuclear power industry releases small quantities of a wide variety of radioactive materials at each stage in the nuclear fuel cycle. For the public the global yearly average dose is 0.008 millisieverts. The type of radioactive materials, and whether they are liquid, gaseous or particulate depends upon the operation of each process. For instance, nuclear power stations release carbon-14 and sulphur-35, which find their way through food chains to humans. Liquid discharges include radioactive materials that people may ingest through fish and shellfish.
The yearly dose to individuals living close to a power plant is small - usually a fraction of a millisievert; doses to people further away are even smaller. Reprocessing nuclear fuel produces higher doses which vary greatly from plant to plant. For the most exposed members of the public, they can be as high as 0.4 millisieverts, but for most of the population they are very much smaller.
World-wide, there are estimated to be four million workers exposed to artificial radiation as a result of their work, with an average yearly dose of about 1 millisievert. Another five million (mostly in civil aviation) have yearly average doses due to natural radiation of 1.7 millisieverts.
Non-nuclear industries also produce radioactive discharges. They include the processing of ores containing radioactive materials as well as the element for which the ore is processed. Phosphorus ores, for instance, contain radium which can find its way into the effluent. A very different industry, the generation of electricity by coal-fired power stations, results in the release of naturally-occurring radioactive materials from the coal. These are discharged to air and transfer through food chains to the population. However, the radiation doses are always low: 0.001 millisieverts or less.
Accidental releases of radioactive materials. Apart from contamination due to the normal operations of the nuclear industry, radioactivity has been widely dispersed accidentally. The most significant accident was at Chernobyl nuclear power station in the Ukraine, where an explosion caused the release of large amounts of radioactivity over a period of several days. Airborne radioactive material dispersed widely over Europe and even further afield. Contamination at ground level varied considerably, being much heavier where rain washed the radioactivity out of the air. Radiation doses therefore varied significantly from normal. More than 100,000 people were evacuated during the first three weeks following the accident. Whole body doses received from external radiation from the Ukrainian part of the 30-km exclusion zone showed an average value of 15 millisieverts. (source OECD, 1995)
Radiation in Consumer Products. Minute radiation doses are received from the artificial radioactivity in consumer goods such as smoke detectors and luminous watches, and from the natural radioactivity of gas mantles. The global yearly average dose is extremely small (0.0005 millisieverts).
Biological Effects of Ionising Radiation
The health effects of radiation may be divided into those that occur early and those that occur late:
Information on the biological effects of ionizing radiation is assembled and published periodically by a number of expert bodies. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) is an inter-governmental Committee made up of prominent scientists from many countries around the world and is charged with assembling, studying and disseminating information on the observed levels and the effects of ionizing radiation, both natural and man-made. The International Commission on Radiological Protection (ICRP) was established nearly 70 years ago, and is an independent, non-governmental group of experts whose recommedations are generally adopted as the basis for national regulations governing radiation exposure.